|
|
May 2: Faraday's MAGNETISM—ELECTRICITY Vol. 30, pp. 61-72
LECTURE V
MAGNETISM—ELECTRICITY
|
|
|
|
| |
I WONDER whether we shall be too deep to-day or not. Remember that we spoke of the attraction by gravitation of all bodies to all bodies by their simple approach. Remember that we spoke of the attraction of particles of the same kind to each other-that power which keeps them together in masses-iron attracted to iron, brass to brass, or water to water. Remember that we found, on looking into water, that there were particles of two different kinds attracted to each other; and this was a great step beyond the first simple attraction of gravitation, because here we deal with attraction between different kinds of matter. The hydrogen could attract the oxygen and reduce it to water, but it could not attract any of its own particles, so that there we obtained a first indication of the existence of two attractions.
|
|
To-day we come to a kind of attraction even more curious than the last, namely, the attraction which we find to be of a double nature-of a curious and dual nature. And I want, first of all, to make the nature of this doubleness clear to you. Bodies are sometimes endowed with a wonderful attraction, which is not found in them in their ordinary state. For instance, here is a piece of shellac, having the attraction of gravitation, having the attraction of cohesion, and if I set fire to it, it would have the attraction of chemical affinity to the oxygen in the atmosphere. Now all these powers we find in it as if they were parts of its substance; but there is another property which I will try and make evident by means of this ball, this bubble of air [a light India-rubber ball, inflated and suspended by a thread]. There is no attraction between this ball and this shellac at present; there may be a little wind in the rooms slightly moving the ball about, but there is no attraction. But if I rub the shellac with a piece of flannel [rubbing the shellac, and then holding it near the ball], look at the attraction which has arisen out of the shellac simply by this friction, and which I may take away as easily by drawing it gently through my hand. [The lecturer repeated the experiment of exciting the shellac, and then removing the attractive power by drawing it through his hand.] Again, you will see I can repeat this experiment with another substance; for if I take a glass rod, and rub it with a piece of silk covered with what we call amalgam, look at the attraction which it has; how it draws the ball toward it; and then, as before, by quietly rubbing it through the hand, the attraction will be all removed again, to come back by friction with this silk.
|
|
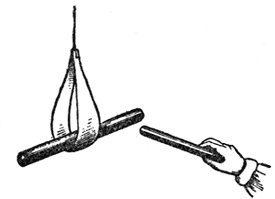
But now we come to another fact. I will take this piece of shellac, and make it attraction by friction; and remember that, whenever we get an attraction of gravity, chemical affinity, adhesion, or electricity (as in this case), the body which attracts is attracted also, and just as much as that ball was attracted by the shellac, the shellac was attracted by the ball. Now I will suspend this piece of excited shellac in a little paper stirrup, in this way (FIG. 33), in order to make it move easily, and I will take another piece of shellac, and, after rubbing it with flannel, will bring them near together: you will think that they ought to attract each other; but now what happens? It does not attract; on the contrary, it very strongly repels, and I can thus drive it round to any extent. These, therefore, repel each other, although they are so strongly attractive-repel each other to the extent of driving this heavy piece of shellac round and round in this way. But if I excite this piece of shellac as before, and take this piece of glass and rub it with silk, and then bring them near, what think you will happen? [The lecturer held the excited glass near the excited shellac, when they attracted each other strongly.] You see, therefore, what a difference there is between these two attractions; they are actually two kinds of attraction concerned in this case, quite different to any thing we have met with before, but the force is the same. We have here, then, a double attraction-a dual attraction or force-one attracting and the other repelling.
|
|
| |
|
|
|
|
|
Fig. 33
|
|
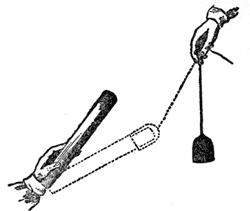
Again, to show you another experiment which will help to make this clear to you: Suppose I set up this rough indicator again [the excited shellac suspended in the stirrup]: it is rough, but delicate enough for my purpose; and suppose I take this other piece of shellac, and take away the power, which I can do by drawing it gently through the hand; and suppose I take a piece of flannel (FIG. 34), which I have shaped into a cap for it and made dry. I will put this shellac into the flannel, and here comes out a very beautiful result. I will rub this shellac and the flannel together (which I can do by twisting the shellac round), and leave them in contact; and then if I ask, by bringing them near our indicator, what is the attractive force? it is nothing; but if I take them apart, and then ask what will they do when they are separated? why, the shellac is strongly repelled, as it was before, but the cap is strongly attractive; and yet, if I bring them both together again, there is no attraction; it has all disappeared [the experiment was repeated]. Those two bodies, therefore, still contain this attractive power; when they were parted, it was evident to your senses that they had it, though they do not attract when they are together.
|
|
| |
|
|
|
|
|
Fig. 34
|
|
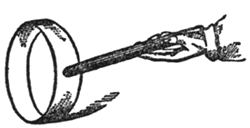
This, then, is sufficient, in the outset, to give you an idea of the nature of the force which we call ELECTRICITY. There is no end to the things from which you can evolve this power. When you go home, take a stick of sealing-wax-I have rather a large stick, but a smaller one will do-and make an indicator of this sort (FIG. 35). Take a watch-glass (or your watch itself will do; you only want something which shall have a round face); and now, if you place a piece of flat glass upon that, you have a very easily moved centre; and if I take this lath and put it on the flat glass (you see I am searching for the centre of gravity of this lath; I want to balance it upon the watch-glass), it is very easily moved round; and if I take this piece of sealing-wax and rub it against my coat, and then try whether it is attractive [holding it near the lath], you see how strong the attraction is; I can even draw it about. Here, then, you have a very beautiful indicator, for I have, with a small piece of sealing-wax and my coat, pulled round a plank of that kind, so you need be in no want of indicators to discover the presence of this attraction. There is scarcely a substance which we may not use. Here are some indicators (FIG. 36). I bend round a strip of paper into a hoop, and we have as good an indicator as can be required. See how it rolls along, traveling after the sealing-wax! If I make them smaller, of course we have them running faster, and sometimes they are actually attracted up into the air. Here, also, is a little collodion balloon. It is so electrical that it will scarcely leave my hand unless to go to the other. See how curiously electrical it is; it is hardly possible for me to touch it without making it electrical; and here is a piece which clings to any thing it is brought near, and which it is not easy to lay down. And here is another substance, gutta-percha, in thin strips: it is astonishing how, by rubbing this in your hands, you make it electrical; but our time forbids us to go farther into this subject at present; you see clearly there are two kinds of electricities which may be obtained by rubbing shellac with flannel or glass with silk.
|
|
| |
|
|
|
|
|
Fig. 36
|
|
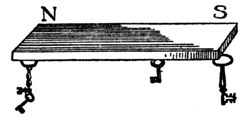
Now there are some curious bodies in nature (of which I have two specimens on the table) which are called magnets or loadstones; ores of iron, of which there is a great deal sent from Sweden. They have the attraction of gravitation, and attraction of cohesion, and certain chemical attraction; but they also have a great attractive power, for this little key is held up by this stone. Now that is not chemical attraction; it is not the attraction of chemical affinity, or of aggregation of particles, or of cohesion, or of electricity (for it will not attract this ball if I bring it near it), but it is a separate and dual attraction, and, what is more, one which is not readily removed from the substance, for it has existed in it for ages and ages in the bowels of the earth. Now we can make artificial magnets (you will see me to-morrow make artificial magnets of extraordinary power). And let us take one of these artificial magnets and examine it, and see where the power is in the mass, and whether it is a dual power. You see it attracts these keys, two or three in succession, and it will attract a very large piece of iron. That, then, is a very different thing indeed to what you saw in the case of the shellac, for that only attracted a light ball, but here I have several ounces of iron held up. And if we come to examine this attraction a little more closely, we shall find it presents some other remarkable differences; first of all, one end of this bar (FIG. 37) attracts this key, but the middle does not attract. It is not, then, the whole of the substance which attracts. If I place this little key in the middle it does not adhere; but if I place it there, a little nearer the end, it does, though feebly. Is it not, then, very curious to find that there is an attractive power at the extremities which is not in the middle-to have thus in one bar two places in which this force of attraction resides? If I take this bar and balance it carefully on a point, so that it will be free to move round, I can try what action this piece of iron has on it. Well, it attracts one end, and it also attracts the other end, just as you saw the shellac and the glass did, with the exception of its not attracting in the middle. But if now, instead of a piece of iron, I take a magnet, and examine it in a similar way, you see that one of its ends repels the suspended magnet; the force, then, is no longer attraction, but repulsion; but, if I take the other end of the magnet and bring it near, it shows attraction again.
|
|
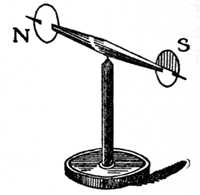
You will see this better, perhaps, by another kind of experiment. Here (FIG. 38) is a little magnet, and I have colored the ends differently, so that you may distinguish one form the other. Now this end (S) of the magnet (FIG. 37) attracts the uncolored end of the little magnet. You see it pulls toward it with great power; and, as I carry it round, the uncolored end still follows. But now, if I gradually bring the middle of the bar magnet opposite the uncolored end of the needle, it has no effect upon it, either of attraction or repulsion, until, as I come to the opposite extremity (N), you see that it is the colored end of the needle which is pulled toward it. We are now, therefore, dealing with two kinds of power, attracting different ends of the magnet-a double power, already existing in these bodies, which takes up the form of attraction and repulsion. And now, when I put up this label with the word MAGNETISM, you will understand that it is to express this double power.
|
|
| |
|
|
|
|
|
Fig. 37
|
|
| |
|
|
|
|
|
Fig. 38
|
|
Now with this loadstone you may make magnets artificially. Here is an artificial magnet (FIG. 39) in which both ends have been brought together in order to increase the attraction. This mass will lift that lump of iron, and, what is more, by placing this keeper, as it is called, on the top of the magnet, and taking hold of the handle, it will adhere sufficiently strongly to allow itself to be lifted up, so wonderful is its power of attraction. If you take a needle, and just draw one of its ends along one extremity of the magnet, and then draw the other end along the other extremity, and then gently place it on the surface of some water (the needle will generally float on the surface, owing to the slight greasiness communicated to it by the fingers), you will be able to get all the phenomena of attraction and repulsion by bringing another magnetized needle near to it.
|
|
| |
|
|

|
|
|
Fig. 39
|
|
I want you now to observe that, although I have shown you in these magnets that this double power becomes evident principally at the extremities, yet the whole of the magnet is concerned in giving the power. That will at first seem rather strange; and I must therefore show you an experiment to prove that this is not an accidental matter, but that the whole of the mass is really concerned in this force, just as in falling the whole of the mass is really acted upon by the force of gravitation. I have here (FIG. 40) a steel bar, and I am going to make it a magnet by rubbing it on the large magnet (FIG. 39). I have now made the two ends magnetic in opposite ways. I do not at present know one from the other, but we can soon find out. You see, when I bring it near our magnetic needle (FIG. 38), one end repels and the other attracts; and the middle will neither attract nor repel-it can not, because it is half way between the two ends. But now, if I break out that piece (n, s), and then examine it, see how strongly one end (n) pulls at this end (S, FIG. 38), and how it repels the other end (N). And so it can be shown that every part of the magnet contains this power of attraction and repulsion, but that the power is only rendered evident at the end of the mass. You will understand all this in a little while; but what you have now to consider is that every part of this steel is in itself a magnet. Here is a little fragment which I have broken out of the very centre of the bar, and you will still see that one end is attractive and the other is repulsive. Now is not this power a most wonderful thing? And very strange, the means of taking it from one substance and bringing it to other matters. I can not make a piece of iron or any thing else heavier or lighter than it is; its cohesive power it must and does have; but, as you have seen by these experiments, we can add or subtract this power of magnetism, and almost do as we like with it.
|
|
| |
|
|
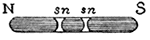
|
|
|
Fig. 40
|
|
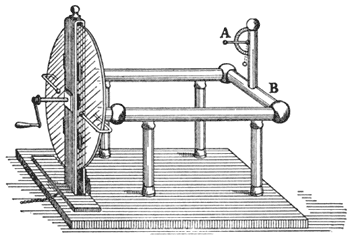
And now we will return for a short time to the subject treated of at the commencement of this lecture. You see here (FIG. 41) a large machine arranged for the purpose of rubbing glass with silk, and for obtaining the power called electricity; and the moment the handle of the machine is turned a certain amount of electricity is evolved, as you will see by the rise of the little straw indicator (at A). Now I know, from the appearance of repulsion of the pith ball at the end of the straw, that electricity is present in those brass conductors (BB), and I want you to see the manner in which that electricity can pass away [touching the conductor (B) with his finger, the lecturer drew a spark from it, and the straw electrometer immediately fell]. There, it has all gone; and that I have really taken it away you shall see by an experiment of this sort. If I hold this cylinder of brass by the glass handle, and touch the conductor with it, I take away a little of the electricity. You see the spark in which it passes, and observe that the pith-ball indicator has fallen a little, which seems to imply that so much electricity is lost; but it is not lost; it is here in this brass, and I can take it away and carry it about, not because it has any substance of its own, but by some strange property which we have not before met with as belonging to any other force. Let us see whether we have it here or not. [The lecturer brought the charged cylinder to a jet from which gas was issuing; the spark was seen to pass from the cylinder to the jet, but the gas did not light.] Ah! the gas did not light, but you saw the spark; there is, perhaps, some draught in the room which blew the gas on one side, or else it would light; we will try this experiment afterward. You see from the spark that I can transfer the power from the machine to this cylinder, and then carry it away and give it to some other body.
|
|
| |
|
|
|
|
|
Fig. 41
|
|
You know very well, as a matter of experiment, that we can transfer the power of heat from one thing to another; for if I pout my hand near the fire it becomes hot. I can show you this by placing before us this ball, which has just been brought red-hot from the fire. If I press this wire to it some of the heat will be transferred from the ball, and I have only now to touch this piece of gun-cotton with the hot wire, and you see how I can transfer the heat from the ball to the wire, and from the wire to the cotton. So you see that some powers are transferable, and others are not. Observe how long the heat stops in this ball. I might touch it with the wire or with my finger, and if I did so quickly I should merely burn the surface of the skin; whereas, if I touch that cylinder, however rapidly, with my finger, the electricity is gone at once-dispersed on the instant, in a manner wonderful to think of.
|
|
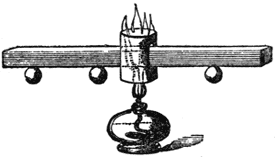
I must now take up a little of your time in showing you the manner in which these powers are transferred from one thing to another; for the manner in which force may be conducted or transmitted is extraordinary, and most essential for us to understand. Let us see in what manner these powers travel from place to place. Both heat and electricity can be conducted; and here is an arrangement I have made to show how the former can travel. It consists of a bar of copper (FIG. 42); and if I take a spirit lamp (this is one way of obtaining the power of heat) and place it under that little chimney, the flame will strike against the bar of copper and keep it hot. Now you are aware that power is being transferred from the flame of that lamp to the copper, and you will see by-and-by that it is being conducted along the copper from particle to particle; for inasmuch as I have fastened these wooden balls by a little wax at particular distances from the point where the copper is first heated, first one ball will fall and then the more distant ones, as the heat travels along, and thus you will learn that the heat travels gradually through the copper. You will see that this is a very slow conduction of power as compared with electricity. If I take cylinders of wood and metal, joined together at the ends, and wrap a piece of paper round, and then apply the heat of this lamp to the place where the metal and wood join, you will see how the heat will accumulate where the wood is, and burn the paper with which I have covered it; but where the metal is beneath, the heat is conducted away too fast for the paper to be burned. And so, if I take a piece of wood and a piece of metal joined together, and put it so that the flame shall play equally both upon one and the other, we shall soon find that the metal will become hot before the wood; for if I put a piece of phosphorus on the wood and another piece on the copper, you will find that the phosphorus on the copper will take fire before that on the wood is melted; and this shows you how badly the wood conducts heat. But with regard to the traveling of electricity from place to place, its rapidity is astonishing. I will, first of all, take these pieces of glass and metal, and you will soon understand how it is that the glass does not lose the power which it acquired when it is rubbed by the silk; by one or two experiments I will show you. If I take this piece of brass and bring it near the machine, you see how the electricity leaves the latter and passes to the brass cylinder. And again: if I take a rod of metal and touch the machine with it, I lower the indicator; but when I touch it with a rod of glass, no power is drawn away, showing you that the electricity is conducted by the glass and the metal in a manner entirely different; and, to make you see that more clearly, we will take one of our Leyden jars. Now I must not embarrass your minds with this subject too much, but if I take a piece of metal and bring it against the knob at the top and the metallic coating at the bottom, you will see the electricity passing through the air as a brilliant spark. It takes no sensible time to pass through this; and if I were to take a long metallic wire, no matter what the length, at least as far as we are concerned, and if I make one end of it touch the outside, and the other touch the knob at the top, see how the electricity passes! It has flashed instantaneously through the whole length of this wire. Is not this different from the transmission of heat through this copper bar (FIG. 42) which has taken a quarter of an hour or more to reach the first ball?
|
|
| |
|
|
|
|
|
Fig. 42
|
|
Here is another experiment for the purpose of showing the conductibility of this power through some bodies and not through others. Why do I have this arrangement made of brass? [pointing to the brass work of the electrical machine, FIG. 41]. Because it conducts electricity. And why do I have these columns made of glass? Because they obstruct the passage of electricity. And why do I put that paper tassel (FIG. 43) at the top of the pole, upon a glass rod, and connect it with this machine by means of a wire? You see at once that as soon as the handle of the machine is turned, the electricity which is evolved travels along this wire and up the wooden rod, and goes to the tassel at the top, and you see the power of repulsion with which it has endowed these strips of paper, each spreading outward to the ceiling and sides of the room. The outside of that wire is covered with gutta-percha; it would not serve to keep the force from you when touching it with your hands, because it would burst through; but it answers our purpose for the present. And so you perceived how easily I can manage to send this power of electricity from place to place by choosing the materials which can conduct the power. Suppose I want to fire a portion of gunpowder, I can readily do it by this transferable power of electricity. I will take a Leyden jar, or any other arrangement which gives us this power, and arrange wires so that they may carry the power to the place I wish; and then placing a little gunpowder on the extremities of the wires, the moment I make the connection by this discharging rod I shall fire the gunpowder [the connection was made and the gunpowder ignited]. And if I were to show you a stool like this, and were to explain to you its construction, you could easily understand that we use glass legs because these are capable of preventing the electricity from going away to the earth. If, therefore, I were to stand on this stool, and receive the electricity through this conductor, I could give it to anything that I touched. [The lecturer stood upon the insulating stool, and placed himself in connection with the conductor of the machine.] Now I am electrified; I can feel my hair rising up, as the paper tassel did just now. Let us see whether I can succeed in lighting gas by touching the jet with my finger. [The lecturer brought his finger near a jet from which gas was issuing, when, after one or two attempts, the spark which came from his finger to the jet set fire to the gas.] You now see how it is that this power of electricity can be transferred from the matter in which it is generated, and conducted along wires and other bodies, and thus be made to serve new purposes, utterly unattainable by the powers we have spoken of on previous days; and you will not now be at a loss to bring this power of electricity into comparison with those which to we have previously examined, and to-morrow we shall be able to go farther into the consideration of these transferable powers.
|
|
| |
|
|

|
|
|
Fig. 43
|
|
|
|